What is contact failure caused by sulfidation and chlorination?
ID: FAQE10097E
update:
Answer
When relays are operated in an atmosphere where sulfide gas or salt damage is likely to occur, mainly under microload switching, sulfidation or chlorination of the contact surface can occur, which increases contact resistance and ultimately leads to contact failure.
Explanation
Sulfidation
Sulfide gas reacts with silver in the contact material to form silver sulfide (Ag2S). *The darker the color changes from light purple to black, the thicker the silver sulfide becomes. Since silver sulfide is an insulator, contact failure may occur if it is used under a microload and with rare switching frequency.


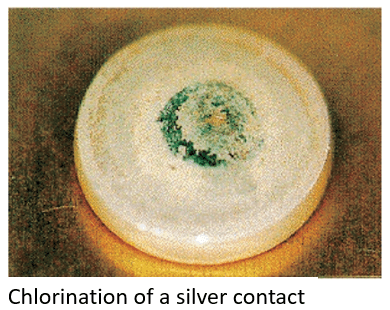
Chlorination
Silver chloride (AgCl, white) is produced in chlorine gas atmospheres or in areas near a coast where salt damage (NaCl) occurs. Contact failure may occur if it is used under a microload and with rare switching frequency.
Regarding to malfunction examples and countermeasure for relays, refer to The SOLUTIONS [General-purpose Relay Edition].
Quick tips
- If you cannot avoid using the relay in an atmosphere of sulfide gas, chloride gas or salt damage, consider using a plastic-sealed relay.
- If the plastic-sealed type cannot be used because of operating conditions such as the switching capacity of the relay, consider countermeasures such as improving the sealing performance inside the housing.
- The smaller the load and the less frequent the switching, the weaker it is against sulfidation and chlorination. Consider measures to destroy the sulfide and chloride coating by increasing the voltage and current applied to the contact.
| Product category | Relays Signal Relays Power Relays |
|---|---|
| Classification | Trouble shootings |
| Related keywords |
|